Most dairy industry meetings over the past year have devoted at least one session or agenda item to safety. DMI’s Innovation Center for U.S. Dairy established a Food Safety Committee almost two years ago to evaluate existing food safety tools and develop new ones. One of the main reasons for this focus was the passage in 2010 of the Food Safety Modernization Act (FSMA).
The Act was the result of consumers and Congress having reached a tipping point of distrust with the entire U.S. food industry’s ability to deliver safe food products. Even though the U.S. dairy industry contributed very little to this tipping point, it was included in the Act because dairy processors deliver key food products to U.S. consumers.
Processors are asking: “Will our current dairy plant operational programs meet FSMA requirements or are additional modification and investment required?” The answer, in general, is that for plants with certification from Safe Quality Foods (SQF), British Retail Consortium (BRC), International Foods Standard (IFS) or Food Safety System Certification 2200 (FSSC), compliance with the FSMA will require only limited additional enhancements of plant operational programs. Dairy plants without one of these certifications may have significant gaps related to FSMA compliance.
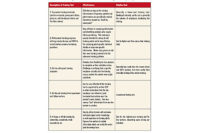
While FDA has still not published regulations for key sections of the FSMA, many provisions were “self-enacting,” meaning that FDA had the authority to enforce immediately after the president’s signature. Fortunately, FDA has shown regulatory restraint in waiting until it published regulations prior to expecting compliance by dairy processors. The table shows key sections of the Act.
Sections 103 and 104 require HACCP-like preventative controls and mandatory plant operational programs (performance standards) and include development of product descriptions, process flow diagrams, hazard analysis, and HACCP Plan Summary tables (HACCP is Hazard Analysis and Critical Control Points.). In addition, processors need written programs supported by records on supervisor and employee training; environmental monitoring and testing; allergen control; employee and processing GMPs; supplier verification for ingredients, packaging and key service providers; and ingredient, packaging and finished product traceability (one step back and one step forward).
Even without regulations, dairy plants need to be sure they have these programs written and in place. FSMA allows FDA to review and copy all written plant programs and their supporting records systems identified above. The actual date when FDA will expect dairy plants to comply with the above-listed items is uncertain, but is expected to range from six months to one year after publication of the regulation.
Other key FSMA tools given to FDA include mandatory recall, administrative detention, fees and penalties. While mandatory recall authority received much attention, administrative detention of dairy products for up to 30 days is likely to have a bigger impact, particularly for dairy products with a short shelf life. Fees of $224 an hour can be assessed against dairy plants if FDA investigators need to re-inspect or “assist” with Class I recalls. Fees can quickly reach thousands of dollars per incident. In addition, plant employees and management can be “penalized” up to $50,000 each (limit of five employees) and the company can be penalized $250,000 by FDA for failure to comply with mandatory recall requirements or other selected sections of the Act.
FSMA applies equally to foreign food processors exporting food ingredients or products to the United States. If U.S. dairy companies import ingredients or dairy products, it is recommended that they demand evidence of conformance with the FSMA. Many of the specific requirements and how FDA will enforce the FSMA in foreign countries have not been made public. It is known that foreign plants will have to register every two years with FDA, they will be inspected through an FDA-accredited private or foreign government auditing organization and be provided a streamlined entry process into the U.S. for additional FDA fees.
While key details of FSMA’s operational requirements for dairy plants are still uncertain, all dairy plants need to make sure they have complied with the sections shown in the table, and ensure their suppliers of ingredients and packaging have done the same. This will smooth many potential bumps in the FSMA compliance road.