What’s scarier than a contamination event? Not planning correctly for reporting to the FDA when your product is involved in one. Fortunately, compliance with the FDA Reportable Food Registry is easier than you think.
That dreaded phone call. That urgent email. None of us likes to think about contamination events, but they are a reality all manufacturers need to plan for. That’s why it’s critical to have a strategy in place to protect your company’s brand and to get your production line back on track as quickly as possible. An important part of your recovery plan is having a record-keeping system in place and knowing how to easily retrieve the right records. How confident are you in the organization of your records? Do you know everything the Food and Drug Administration will require from you should a contamination event occur? How quickly can you compile a report? Your answers to these questions will determine whether you feel a churning in the pit of your stomach or if your in-order, mental checklist exudes confidence to your staff.
As part of its Reportable Food Registry (RFR), the FDA requires manufacturers to report any products that have left your plant that could potentially cause illness to consumers. These reports are made through an electronic portal, opened by the FDA in September 2009. Reports must be submitted to the FDA within 24 hours of learning that a contaminated product has left your facility. These reports may be primary, meaning you notify the FDA that your product has been contaminated; or subsequent, meaning you are reporting contaminated products received from a supplier. Likewise, your recipients could report that products received from you are contaminated in some way. Because of this requirement, even if you are confident that all products leaving your facility were clean and contamination-free, you may need to produce detailed records of your quality testing for the FDA.
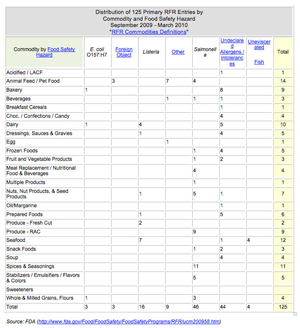
While the RFR adds extra work for manufacturers, it was mandated by Congress to help the FDA better protect consumers by tracking patterns of food and beverage adulteration and by targeting the agency’s limited inspection resources. The first reporting period of the RFR was September 2009 through March 2010. During this time, 125 contamination incidents were reported across 25 commodities and fell into seven categories of contamination: E. coli, foreign object, listeria monocytogenes, salmonella, undeclared allergens/intolerances, and others. Additionally, the RFR collected a subsequent 1,638 reports from recipients of these declared foods and ingredients.
How can you ensure your records are in order and help your company avoid contamination events altogether?
First, continue to do everything you can to ensure the safety of your products. Of course, this is your goal already though, right? But, if you are like many food and beverage manufacturers, you may be feeling pressure to get your products out the door as quickly as possible. This means you’re releasing lots from your facility before all quality testing is complete. Some people call this the “ship and pray” method. You ship your products and then test the lots that have already left your site, knowing that 99% of the time they will be clean and you won’t have a problem. But what happens when there is a problem? Panic strikes. If your products have left your facility, they must be recalled-whether they are on trucks moving around the country or, even worse, on shelves and in the homes of your consumers. And, to be in compliance with the RFR, you must report the event to the FDA within 24 hours.
While the FDA requires reporting on all contaminated products that leave your facility, it does not require contaminated products to be reported if they have not yet left the plant, as they do not pose a risk to consumers if they never leave your facility. This makes it all the more important to test your products before releasing them. But how do you do that without slowing your time to market?
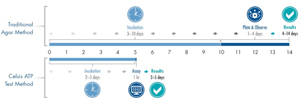
Of the 125 contamination incidents reported through the RFR, 15% were microbial in nature. This statistic can be reduced through the adoption of fast, reliable testing methods. While traditional microbial testing methods can add four to 14 days to your production cycle, a Celsis Rapid Detection system provides reliable microbiological quality results in just two to five days. By removing days from your product screening process, you will be able to hold each lot until you have confirmation that it is free of microbiological contamination. By implementing the right rapid microbial method (RMM) for your company, you can reduce your risk of a public recall, knowing that only safe products will be released from your plant to your customers, and by having your test results readily available to document your process.

The FDA’s regulations on the EMR require a detailed list of information, including but not limited to, the name and contact information for the source of each ingredient, dates ingredients were received, quantity and type of packaging of these ingredients, details about the transportation companies that delivered the ingredients to you and transported the products to your recipients, dates your products are released and more. These records must be created for all lots that you manufacture and must be held for as long as two years and kept organized so that you are able to provide them to the FDA in the event of a contamination at any point in the production cycle.
These regulations can be quite overwhelming for food and beverage manufacturers, but help is available. Although one of the primary benefits of RMMs may be increasing the speed at which you can release your products safely, they can also provide valuable assistance in complying with FDA record-keeping requirements.
The Celsis Rapid Detection system includes a fully customizable database that allows for storage of records related to quality micro testing and provides query and reporting capabilities to help you quickly and easily access specific data. This database allows results to be exported as Excel files to facilitate simple data sharing, and can even be configured for remote access. Results are secure and protected, as the database can be installed on a networked server allowing for scheduled backup. Additionally, the software that supports the Celsis CellScan Innovate system used for dairy, food and beverage products, called Innovate.im, supports you in your efforts to comply with 21CFR through features such as password security, protected data, easy search capabilities, report exporting, record logging and more.
In addition to reducing your production cycle by several days and allowing you to further ensure the quality and safety of your products before they are released, RMMs also simplify and streamline your record-keeping process. These significant benefits also contribute to lean manufacturing practices, including reduced working capital requirements due to shortened cycle times, improved return on invested capital and minimized warehouse space requirements.
If you are involved in a contamination event, the right RMM system can help you not only report the incident to the FDA quickly and accurately, but also physically recover from the event faster. The rapid results provided by RMMs detect contamination earlier in the process, while your product is still held in your warehouse, allowing you to quickly identify the source of the contamination, take corrective action, pull the affected products before they are released into distribution and get your production cycle back up and running fast.
While thinking about contamination events may put you into a cold sweat, don’t let fear of what could happen keep you from planning for the unexpected. Whether you are new to the idea of RMMs or already have a rapid microbial detection system in place, take time today to make sure you are ready for that request for records from the FDA. Investigate the different features of RMMs and see how they could help you get your products to your customers quickly and safely, while reducing your chances of ever having to submit a report to the RFR.
Look at your record-keeping system and think about how a more secure, organized system could simplify your compliance with track and trace requirements. You can’t always predict when you might get that dreaded notice from the FDA, but RMMs can help you establish a strong program today that helps your company respond in the fastest and best way possible.