The application of basic aseptic principles into the design and operation of a plant, along with a packaging machine that utilizes ELL technologies, should, when run properly, give you the shelf life you require.
Shelf life defined
Shelf life, which is always expressed in days, has many definitions depending on the product’s intended use.
For most low acid refrigerated products (pH > 4.6), shelf life can be categorized as the time period before the quality of a perishable product could potentially become a public health risk. Food safety is critical in these types of products.
A secondary component is the time period before the product becomes undesirable for sale or consumption due to factors not related to food safety.
For high acid refrigerated products (pH < 4.6), shelf life can be categorized as the time period before the quality of a perishable product becomes undesirable for sale or consumption.
Shelf life is determined by a number of factors depending on product type.
Microbiological development by far has the greatest effect on determination of shelf life for a low acid product.
Other factors such as organoleptic (flavor, odor, etc.) factors, enzyme breakdown, and vitamin degradation can be important in determining shelf life. This is especially important if the product makes a nutritional or other claim on the label.
Other chemical reactions that could cause the finished product to be undesirable must be considered. It is critical that the finished product adhere to all local and national regulations. Milk processing, (the product focus of this paper) must adhere to FDA-regulated dairy standards including the Pasteurized Milk Ordinance (PMO).
Critical factors for shelf life extension include raw product quality, processing time and temperatures, intermediate plant storage and distribution conditions, packaging environment and retail distribution storage environment.
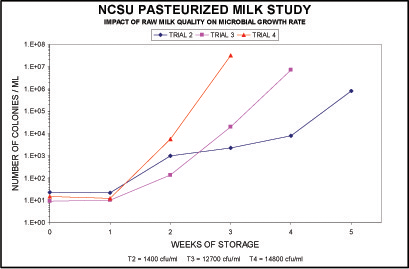
The quantity, type and activity of microbiological organisms contained in raw milk prior to processing and packaging have a direct impact on the shelf life and flavor of the end product.
The following graph shows that when microbiological counts are high, shelf life length is difficult to keep in control consistently.

Enhancing shelf life
The application of basic aseptic principles, including ultra high temperature (UHT) processing, will improve a refrigerated product’s shelf life. Adequate processing time/temperature relationships have to be determined to give the producer the required shelf life. There are some government agency regulated labeling standards that require a mandatory minimum process in order to display the desired label on the package (ex: ultra-pasteurized), but at times it might be necessary to implement a higher process to yield the required shelf life of the finished product.Processing systems should be designed to aseptic standards even though the product is refrigerated. Equipment design should be to the highest sanitary standards and be easily cleaned and sterilized utilizing traditional clean-in-place/sterilize-in-place methods, along with hand cleaning of non-CIP machine surfaces.
Post process contamination is a major cause of reduced shelf life. This can be caused by poor sanitation in the fill system, operator re-contamination from non-sterile hands and tools and an environment (air) that isn’t clean and isn’t low in moisture.
Intermediate storage and distribution systems in the plant must be designed and maintained to aseptic conditions. Surge tanks should be designed to aseptic standards with controlled sterile air venting, the ability to SIP the tank utilizing steam or chemicals and the ability to CIP using traditional methods. Controls should exist around the tank to adequately control and record all functions of the tank.
Pumping, valving and piping systems must also be designed to aseptic standards. Pumps should be designed to prevent product recontamination through the rotary seal. Valving must have a barrier, whether it is a diaphragm, steam or other material, preventing recontamination of product as the valve cycles during operation. Piping systems, which are often overlooked, should run under positive pressure. When the application calls for a line to be under vacuum conditions, care must be taken to secure potential ingress areas from contaminating the product. Piping systems must eliminate "dead legs" where product can reside for extended periods of time.
ELL Filling Technology
The processor must remember that a packaging machine can only preserve the microbiological state of the product it is given; it cannot increase the quality or shelf life of the packaged product. Packaging equipment equipped with extended long life (ELL) design features will further protect the product’s integrity.Extended life technology has similar but different challenges than aseptic technology and can actually be more difficult to implement. Aseptic processing is governed by strict regulations and very black and white: either the product is sterile or it is not.
Extended life production does not have to meet the same strict regulations that aseptic technology does. In fact, except for the labeling standard that governs the term “Ultra-Pasteurized,” extended life production only needs to meet standard Good Manufacturing Practices (GMP) that govern all food plants. While the ultra pasteurized product is close to aseptic standards, it is not considered to be commercially sterile. There is the potential to have different types of organisms at low levels in the finished product.
An automatic sanitizing system is an important extended life technology that maintains control of all of the places where contamination may occur. Carton contact areas including mandrels and other parts of the infeed and filling sections are periodically sprayed with sanitizing solution to control organisms that might deposit on the surface from the environment.
Minimizing condensation is critical to the success of a long life packaging machine. Condensate control minimizes the formation of condensate on various surfaces of the machine that must be cooled. Good CIP procedures and pre-production sanitation must be practiced by the processor, along with hand cleaning of non-CIP machine surfaces.
Good environmental controls also are critical in a packaging machine. A HEPA (High Efficiency Particulate Air) system utilizes a system of filters that are 99.97% effective upon particles that are at least 0.3 microns in size. Dual HEPA barriers between the inside and outside environment deliver “a clean room within a clean room” for further protection. Using UV sanitizing technology on the HEPA air distribution system further enhances the hygiene of the machine.
To ensure package sanitation is maintained, it is best to minimize human handling of blank packages. Storing carton blanks in a clean environment also is essential. Operators and maintenance personnel need to be constantly aware of their effect on machine sanitation. Repeated hand sanitizing or wearing sanitized gloves should be required. When adjustments are made to the machine, sanitizing steps should be performed to assure the machine is not compromised in any way.
The inside of the cartons must be sanitized prior to filling using aseptic technology to ensure they will be organism free prior to filling. A combination of 35% hydrogen peroxide along with sterilized hot air to activate and vaporize it delivers at least a 4D kill of bacteria. Peroxide is also applied to the carton closure.
Impact of storage temperature
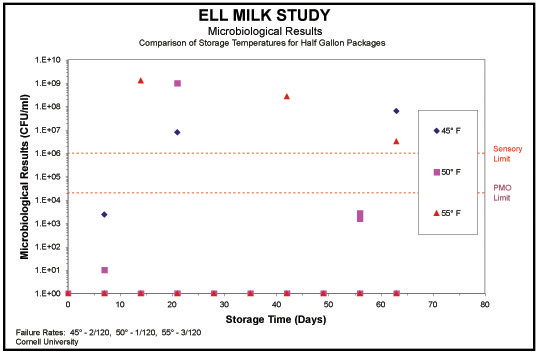
Distribution and retail environment storage temperature is probably the most critical for preserving shelf life. Lower storage temperatures extend the microbial shelf life of dairy products that are processed under normal pasteurization conditions. For a standard pasteurized dairy product, every 5°F increase in the average storage temperature cuts the expected shelf life in half. Maximum filling temperatures of 35-38°F are recommended to meet regulatory requirements and reach the longest potential shelf life.
Storage temperature appears to have less of an impact on Ultra-Pasteurized processed and ELL packaged milk. Studies indicate there was no statistical difference in the shelf life of ELL product held at 45°, 50°, and 55°F. Of course, regulations require a storage temperature of 45OF, and although it is not recommended, ELL can assist a producer in a broken cold chain or temperature abuse situation where 45° is not maintained for short periods of time.
Other ELL benefits
In addition to all of these product integrity advantages an ELL packaging machine technology provides, it is expected to deliver many production and marketing benefits, including increased plant efficiency through longer production runs, reduced distribution costs with fewer, larger, deliveries to distant markets, reduced product returns from expired, short-term pull dates, increased retail facings due to longer pull dates which boost consumer purchases, increased sales (consumers equate longer pull dates with freshness) and enhanced brand building.
The application of basic aseptic principles into the design and operation of a plant, along with a packaging machine that utilizes ELL technologies, should, when run properly, give you the shelf life you require.
Richard J. Szyperski, technical product manager
Evergreen Packaging Equipment, Cedar Rapids, Iowa